Real-time quantitative dynamic dosimetry optimization during a prostate brachytherapy procedure would allow the physician to account for changes and deviations from the original plan and thus tailor the radiation dose to cancer without harming surrounding healthy tissue. Dynamic dosimetry has been demonstrated to improve dosimetric quality and thus promises to lead to improved clinical outcomes. While the concept has been known for nearly a decade, there is no solution that would be applicable in community practice, outside the confines of luminary research hospitals, primarily due to the complexity and cost of current approaches for dynamic dosimetry.
The objective of this research is to design, develop, and evaluate a method for intra-operative localization of the implanted seeds in relation to the prostate, to allow for in-situ dosimetric optimization and exit dosimetry.
We fuse ultrasound and fluoroscopy imaging by purely computational means, without auxiliary gadgetry. Generally, the fusion process requires three steps: (a) estimate of the relative poses of the individual fluoroscopy images, (b) reconstruct the location of implanted seeds in the coordinate frame of the fluoroscope, and (c) register the implanted seeds and the ultrasound images of the prostate. Following this, the remainder of the implant plan can be easily re-optimized and the operating physician would instantaneously correct any deviation from optimum dosimetry, during the procedure in the operating room.
Pose estimation of fluoroscopy images
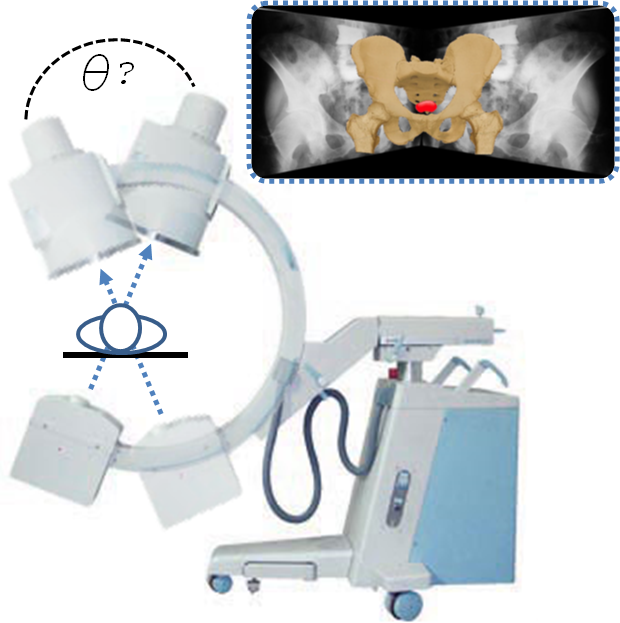
C-arm pose encoding using IMU sensor
C-arm fluoroscopes are ubiquitous in computer-assisted interventions. They are versatile, compact, and mobile real-time X-ray imaging devices. The basic use of a C-arm is to acquire 2D X-ray images that can be reconstructed into three-dimensional representation. The reconstruction process requires that the relative poses (angles) of the 2D projection images must be known. Accurate, practical, and affordable C-arm pose tracking is a major technical challenge in using manually operated and un-encoded conventional C-arm fluoroscopes. One such application is the 3D reconstruction of implanted radioactive seed for prostate cancer brachytherapy. X-ray images are taken of the prostate at different angles using the C-arm and then using these images a 3D reconstruction of the implanted seeds is created. This provides a visualization of the distribution of radioactive seeds to determine if the treatment plan was successful. However, the procedure has constraints imposed by potential collisions with patient, operating table, and standard brachytherapy instrumentation create a narrow operating range for the C-arm. These constraints hamper existing tracking methods from becoming clinically viable solutions. Our new tracking method makes use of an accelerometer that is affordable, compact, and practical for encoding the rotational pose of a C-arm. By configuring the accelerometer as a tilt (angle) sensing device, we are able to construct a series of equations that accurately and precisely track the rotational pose of a full scale fluoroscopic C-arm.
C-arm pose encoding using X-ray fiducial
Prostate brachytherapy is performed with transrectal ultrasound guidance that provides adequate real-time visualization of the prostate but not of the implanted seeds. At the same time, C-arm fluoroscopy is widely used for visual assessment and 3D reconstruction of the implanted seeds, but it cannot show the prostate and other relevant structures. Fusion of the two modalities would enable intraoperative implant optimization, which requires tracking of the C-arm poses. Pose recovery on C-arm machines is a major technical problem that presently does not have a clinically practical solution in many areas of application. The relative poses of fluoroscopy images are determined in one of following three ways: (i) electronic joint encoders, (ii) optical or electromagnetic tracker, and (iii) radiographic fiducials. Fully encoded C-arms are very expensive and thus virtually non-existent in brachytherapy. External trackers are also impractical for various reasons and also add costs. Optical tracking requires line of sight which imparts alterations in clinical setup and workflow. Electromagnetic tracking overcomes these issues, but it is susceptible to field distortion.
Registration of Ultrasound to Fluoroscopy (RUF)
Develop methods for reconstruction of seed implants from X-ray fluoroscopy and spatially register them to the prostate anatomy identified in TRUS.
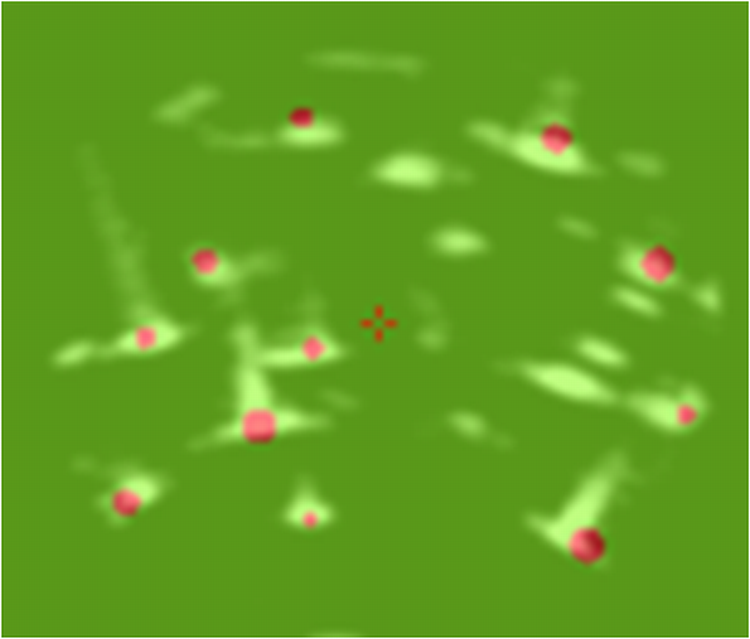
The relative C-arm pose along with segmented seed information is fed to the algorithm we have developed (MARSHAL) that reconstructs the 3D locations of implanted seeds from three (or more) X-ray images. The reconstructed implant configuration is projected into ultrasound space where it can be superimposed on the prostate boundary.
Additional research is conducted in quantifying the effects of TRUS and fluoroscopy registration error on dosimetric quality. Specifically, we analyze the effect of each translational and rotational degree of freedom in the registration error on dose volume and dose surface histograms. Also, we developed methods to account for C-arm pose measurement errors in order to reconstruct seeds in 3D using fluoroscopy. Lastly, as an alternative to initialize the intensity based registration for dynamic dosimetry, we identify 3D needle tracks from fluoroscopy images and perform a needle/needle ultrasound to fluoroscopy registration.
We perform pre-clinical laboratory testing to validate the accuracy, consistency, and robustness of ultrasound-fluoroscopy registration and seed implant reconstruction. In particular, we will first conduct workspace and accuracy analysis on images of appropriately fabricated prostate phantoms, and then on pre-recorded brachytherapy cases of real patients.
Tomosynthesis-based brachytherapy seed reconstruction and dosimetry evaluation
One way to ensure quality of the operation intra-operatively is to reconstruct the implanted seeds into a 3D space using a few fluoroscopic images, hence obtaining every seed’s spatial coordinate. The delivered dose to the prostate tissue and DVH are then calculated and compared to the preoperative plan.
However there are some difficulties associated with reconstructing seeds using fluoroscopic projection images including distortion in the X-ray images, limited C-arm rotational mobility around the AP-axis, hidden seeds and overlapping seeds in images. Hence it would be interesting to develop a method which eliminates the mentioned difficulties, making the seed reconstruction procedure more accurate and reliable. Also it would be interesting to evaluate the dosimetry delivered to the prostate tissue when the seed’s spatial coordinates are subject to error due to error in C-arm pose extraction and distortion in X-ray images.
Brachytherapy ultrasound calibration

The intrinsic quality (i.e. the accuracy) of a brachytherapy system depends on the accuracy of its calibration. Inaccurate system calibration causes faulty needle and radiation source placement, which may result in insufficient dose to the cancer and/or inadvertent radiation of nearby sensitive organs, the rectum, urethra and bladder.
In current brachytherapy practice, calibration is a laborious manual process, performed periodically outside the operating room, with the assumption that the resulting calibration parameters remain valid over time. But during storage, transportation and setup in the operating room, calibration parameters may change inadvertently. Calibration errors are practically invisible in the operating room, thus the physician can never be certain whether the system will function accurately in the operating room.
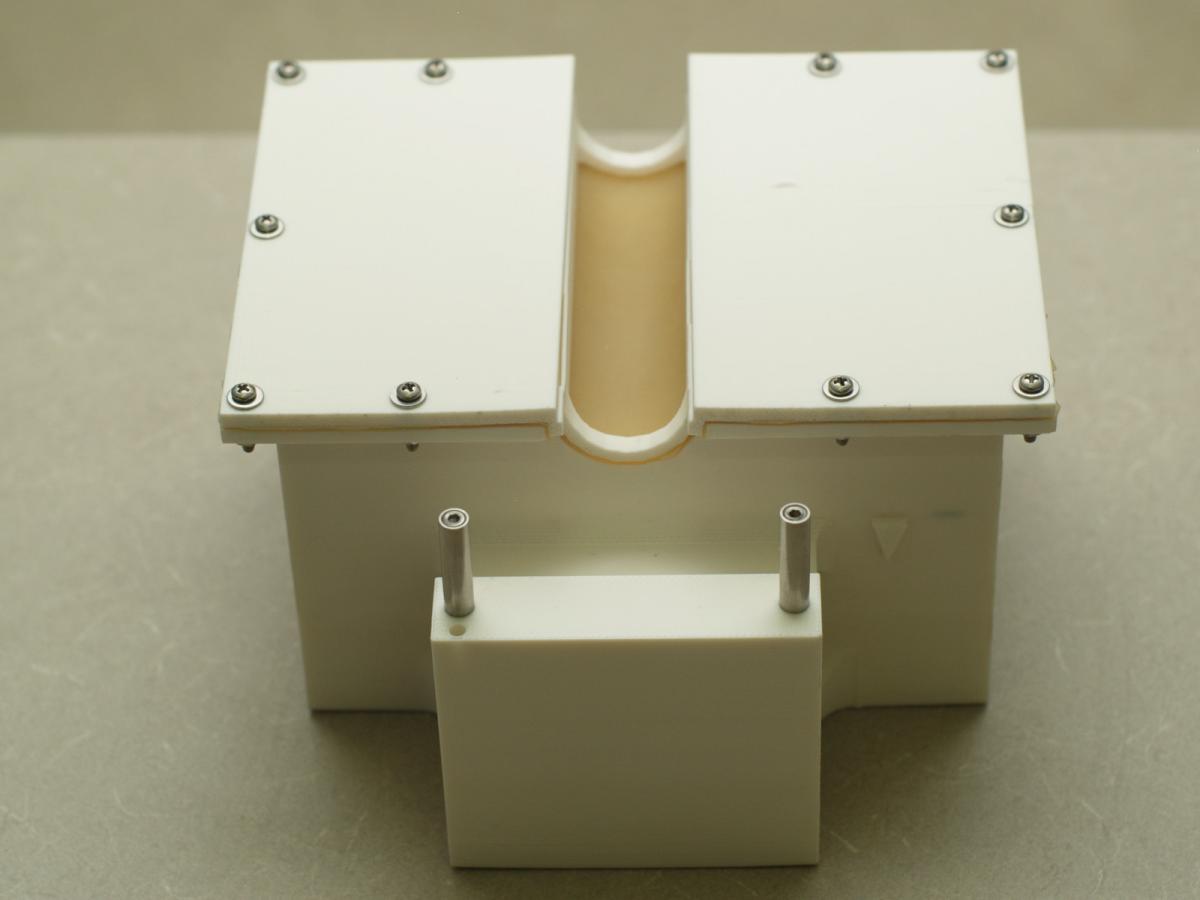
Our goal is to develop automated computational calibration for prostate cancer brachytherapy. The calibration will be performed in the operating room as the patient is prepared for surgery. Our invention eradicates the current practice of pre-operatively performed, labor-intensive and subjective manual calibration processes, increasing the safety and accuracy of all prostate cancer brachytherapy systems.