Prostate motion analysis
Transrectal ultrasound (TRUS) is currently the standard imaging modality for guiding biopsy due to its low cost and ease-of-use.3 However, because of the poor image quality of ultrasound, TRUS only has a detection rate of 20-30%. Many studies have shown that this method misses the cancer in at least 20% of the cases.5-7 Magnetic resonance imaging (MRI) provides an alternative approach to the detection and diagnosis of prostate cancer. MRI has high spatial resolution, excellent soft tissue contrast, and volumetric imaging capabilities.
Closed-bore MRI has confined physical space; therefore such approach requires robotic assistance. Krieger et al. developed an MRI-guided robotic prostate biopsy system in 2003. Since then, over 200 biopsies were performed with this system by the U.S. National Cancer Institute (NCI). The robot guides a biopsy needle through the rectum into the targeted locations within the prostate to collect tissue samples. However, due to patient motion and organ dislocation during the procedure, the needle does not always reach the targeted region.
The goal of the project is to perform a quantitative evaluation of the clinical accuracy for this MRI-guided robotic biopsy system.
Intra-operative prostate tracking in MRI-guided biopsy
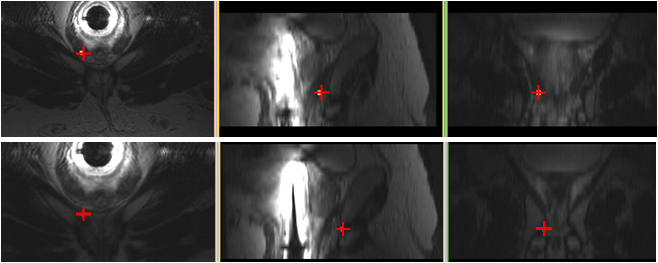
Magnetic Resonance Imaging (MRI) is increasingly becoming the modality of choice in prostate biopsy. During a prostate biopsy procedure, the prostate moves between needle insertions. We propose a prostate tracking algorithm under MR imaging by multislice-to-volume registration. Multiple orthogonal slices acquired after each needle insertion are registered to a high-resolution target planning volume. Six degree of freedom motion - translation along and rotation about each of the three axes - is recovered by the algorithm and the new coordinate position of the biopsy target is computed to make the proper adjustments for the next needle insertion. The figure below shows the change in target biopsy position between needle insertions for one of the patient data sets. The top image shows the biopsy target position before the tracking adjustment (the originally planned target) and the bottom image shows the biopsy target position after the tracking adjustment (registration) as computed by the algorithm. The bottom image indicates the correct position in which to insert the needle for core extraction.
Prostate registration evaluation
The biopsy and localized therapy of prostate cancer involves needle placement under image guidance. The typical workflow begins with extensive pre-operative (pre-op) diagnostic imaging and based on these images the biopsy or therapy target locations are identified. During the procedure, intra-operative (intra-op) images are acquired to determine the current position of the planned targets and then to verify the accuracy of needle placement relative to the target anatomy. Transforming pre-op targets into intra-op imagery is a supremely difficult task, due to incessant organ deformation, dislocation, and inherent differences between the pre-op and intra-op imaging modalities. A substantial arsenal of methods has been developed to register pre-op and intra-op prostate images, involving just about all combinations of popular prostate imaging modalities: ultrasound, MRI, CT, and cone beam fluoroscopy. Validation and quantitative comparison of the competing approaches require a large number of images with known ground truth for prostate and internal target locations. Acquiring lots of high- quality clinical images is prohibitively expensive and, more often than not, infeasible intra-operatively. In short, despite decades of research in image-guided surgery, ground truth target dislocation information in clinical imagery is virtually nonexistent. Even under the very best of circumstances, information is limited to surrogates, such as manually segmented contours and anatomical landmarks. The overarching goal of our project is to create a simulation environment to generate realistic prostate imaging data in a controllable way, with known ground truth, with the primary purpose of serving the evaluation, comparison, and optimization of target registration methods, specifically in image-guided prostate interventions.
